Search
Guideline 12.2 – Paediatric Advanced Life Support (PALS)
Next review July 2024
Summary

ANZCOR Guidelines 12.1 to 12.5 are provided to assist health professionals in the resuscitation of children. Differences from the adult and newborn guidelines reflect differences in the causes of cardiorespiratory arrest in, and anatomy and physiology of newborns, older infants, children and adults. These guidelines draw from Paediatric Life Support 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations1 the development of which included representation from ANZCOR. The 2020 European Resuscitation Council Paediatric Life Support guidelines2, 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Care3, previous Paediatric Life Support International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations4-6 statements and local practices have also been taken into account.
ANZCOR Guideline 12.2 focuses on paediatric resuscitation in healthcare settings (pre-hospital or hospital) by health professionals responsible for the care of infants and children. It should be read in conjunction with the other paediatric guidelines (ANZCOR Guidelines 12.1 to 12.5) and the general life support guidelines suitable for all age groups (ANZCOR Guidelines 2 to 8).
To whom does this guideline apply?
This guideline applies to infants and children requiring advanced life support (PALS) in a healthcare environment (pre-hospital or hospital).
Who is the audience for this guideline?
This guideline is intended for health professionals who care for infants and children in healthcare environments where resuscitation equipment and medications are available.
It represents the next steps in the continuum of care from bystander BLS and/or health professional paediatric basic life support (PBLS) through to use and availability of more advanced skills and resources allowing provision of paediatric advanced life support (PALS).
Summary of Recommendations
The Australian and New Zealand Committee on Resuscitation (ANZCOR) makes the following recommendations:
- ANZCOR suggests that paediatric rapid response systems - medical emergency teams (MET) or rapid response teams (RRT) – should be implemented in all hospitals that care for children [CoSTR 2015, weak recommendation, very low quality of evidence].
- ANZCOR suggests that palpation of a pulse (or its absence) is not reliable as the sole determinant of cardiac arrest and need for chest compressions. In infants and children who are unresponsive and not breathing normally, healthcare providers should begin CPR unless they can definitely feel a pulse within 10 seconds [CoSTR 2020].
- ANZCOR suggests that the highest concentration of oxygen available (100%) should be administered during initial resuscitation regardless of any preceding condition. [Good Practice Statement].
- ANZCOR suggests that when using airway opening manoeuvres, if a neck injury is suspected, jaw thrust should be used to avoid worsening the injury [Good Practice Statement].
- ANZCOR suggests that two initial ventilations be provided before commencement of chest compressions because asphyxial causes are more common than cardiac causes in paediatric cardiopulmonary arrest. In circumstances where the usual equipment used to provide ventilations (eg. bag-valve-mask equipment) is not immediately available, CPR should be commenced immediately with chest compressions [CoSTR 2015].
- ANZCOR suggests that rescuers provide ventilations and chest compressions for pediatric in-hospital cardiac arrest (IHCA) and out-of-hospital cardiac arrest (OHCA) [CoSTR 2020, weak recommendation, very low quality of evidence]. If rescuers are unable or unwilling to provide ventilations, they should at least perform chest compressions [CoSTR 2020, Good Practice Statement].
- ANZCOR suggests the use of bag-valve-mask (BVM) ventilation rather than endotracheal tube (ETT) or supraglottic airway (SGA) insertion in the management of children during cardiac arrest in the out-of-hospital setting [CoSTR 2019, weak recommendation, very low certainty of evidence]. There is insufficient evidence to support any recommendation about the use of ETT or SGA in the management of children with cardiac arrest in the in-hospital setting.
- ANZCOR suggests that both cuffed and uncuffed tracheal tubes are acceptable for use in infants and children undergoing emergency intubation. If tracheal tubes are used, avoid excessive cuff pressures [CoSTR 2010, LOE V].
- ANZCOR suggests that, if cricoid pressure is used during emergency intubation in infants and children, it should be discontinued if it impedes ventilation or interferes with the speed or ease of intubation [CoSTR 2010].
- ANZCOR suggests that confirmation of tracheal tube position using exhaled CO2 detection (colorimetric detector or capnography) should be used for intubated infants and children with a perfusing cardiac rhythm in all settings (eg. out-of-hospital, emergency department, intensive care unit, inpatient, operating room). In infants and children with a perfusing rhythm, it may be beneficial to monitor continuous capnography or frequent intermittent detection of exhaled CO2 during out-of-hospital and intrahospital or interhospital transport. [CoSTR 2005].
- ANZCOR suggests that, after placement of a secure airway, avoid hyperventilation of infants and children during resuscitation from cardiac arrest, whether asphyxial or arrhythmic in origin. A reduction in minute ventilation to less than baseline for age is reasonable to provide sufficient ventilation to maintain adequate ventilation-to-perfusion ratio during CPR while avoiding the harmful effects of hyperventilation. There are insufficient data to identify the optimal tidal volume or respiratory rate [CoSTR 2020, Good Practice Statement].
- ANZCOR suggests that rescuers compress the chest by at least one third the anteroposterior dimension, or approximately 4 cm in an infant and 5cm in a child [CoSTR 2015, weak recommendation, very low-quality evidence].
- ANZCOR suggests that in the setting of cardiac arrest, the intraosseous route is recommended if peripheral or central venous access is not already in place [CoSTR 2010, LOE III-1].
- ANZCOR suggests the following in relation to calculation of medication and fluid doses in paediatric resuscitation [CoSTR 2010, LOE V]:
-
- To calculate the dose of resuscitation medications, use the child’s weight if known. If the child’s weight is unknown, it is reasonable to use a body length tape with precalculated doses.
- In non-obese pediatric patients, initial resuscitation medication doses should be based on actual body weight (which closely approximates ideal body weight). If necessary, body weight can be estimated from body length.
- In obese patients, the initial doses of resuscitation medications should be based on ideal body weight that can be estimated from length. Administration of medication doses based on actual body weight in obese patients may result in medication toxicity.
- Subsequent doses of resuscitation medications in both non-obese and obese patients should take into account the observed clinical effects and toxicities. It is reasonable to titrate the dose to the desired therapeutic effect, but it should not exceed the adult dose.
-
- ANZCOR suggests that the initial dose of adrenaline (epinephrine) in paediatric patients with non-shockable rhythm IHCA) and OHCA be administered as early in the resuscitation as possible [CoSTR 2020, weak recommendation, very low-certainty evidence].1 In the absence of consistent evidence regarding the optimal interval for subsequent adrenaline (epinephrine) doses in paediatric patients with IHCA or OHCA, ANZCOR suggests that the current recommended practice of administration at intervals of 3-5 minutes (or every second loop of the PALS pathway) continues [ANZCOR 2020, Good Practice Statement].
- ANZCOR suggests that administration of sodium bicarbonate is not used in the management of pediatric cardiac arrest. It has a specific role in hyperkalaemia and arrhythmias associated with tricyclic antidepressant overdose [CoSTR 2010].
- ANZCOR suggests that self-adhesive defibrillation pads are used in infants and children in cardiac arrest. The largest size pads that fit an infant’s or child’s chest without touching each other should be used [CoSTR 2010].
- For the sake of simplicity, ANZCOR continues to suggest 4 J/kg for the initial and subsequent doses (ie. all shocks) of unsynchronised shock for VF and pulseless VT, followed immediately by 2 minutes of CPR without waiting to analyse the rhythm [CoSTR 2015, weak recommendation, very-low-quality evidence].
- There is currently insufficient evidence to recommend for or against the routine use of bedside ultrasound and echocardiography during a paediatric arrest. Ultrasonography may be considered to identify potentially treatable causes (4H4T) of an arrest when appropriately skilled personnel are available, but the benefits must be carefully weighed against the known deleterious consequences of interrupting chest compressions [CoSTR 2020].
- ANZCOR suggests the use of real-time audiovisual feedback and prompt devices during CPR in clinical practice as part of a comprehensive quality improvement program for cardiac arrest designed to ensure high-quality CPR delivery and resuscitation care [CoSTR 2020, weak recommendation, very-low-certainty evidence]. ANZCOR suggests against the use of real-time audiovisual feedback and prompt devices in isolation (ie. not part of a comprehensive quality improvement program) [CoSTR 2020, weak recommendation, very-low-certainty evidence].
- ANZCOR suggest that family members of patients undergoing resuscitation should be given the option to be present, ideally with an assigned support person. Each healthcare institution should have a family presence policy and staff education strategy in place [Good Practice Statement].
Abbreviations
|
Abbreviation |
Meaning/Phrase |
|
AED |
automated external defibrillator |
|
ALS |
advanced life support |
|
ANZCOR |
Australian and New Zealand Committee on Resuscitation |
|
ARC |
Australian Resuscitation Council |
|
BLS |
basic life support |
|
BVM |
bag-valve-mask |
|
CoSTR |
Consensus on Science with Treatment Recommendations |
|
CPR |
cardiopulmonary resuscitation |
|
ETT |
endotracheal tube |
|
IHCA |
in-hospital cardiac arrest |
|
ILCOR |
International Liaison Committee on Resuscitation |
|
IO |
intraosseus |
|
IV |
intravenous |
|
LOE |
Level of Evidence |
|
NZRC |
New Zealand Resuscitation Council |
|
OHCA |
out-of-hospital cardiac arrest |
|
PALS |
paediatric advanced life support |
|
PBLS |
paediatric basic life support |
|
PEA |
pulseless electrical activity |
|
pVT |
pulseless ventricular tachycardia |
|
RCT |
randomised control trial |
|
ROSC |
return of spontaneous circulation |
|
SGA |
supraglottic airway |
|
VF |
ventricular fibrillation |
Introduction

This guideline is provided by ANZCOR to assist health professionals responding to significant clinical deterioration or cardiorespiratory arrest in infants and children in healthcare settings. For health professionals caring for both adults and children, this guideline should act as an adjunct to the Advanced Life Support (ALS) guidelines for adults published by ANZCOR (Section 11). Differences between paediatric and adult guidelines reflect differences in the causes of cardiorespiratory arrest and differences in anatomy and physiology between infants, children and adults. Differences from paediatric basic life support (PBLS) recommendations (Refer to ANZCOR Guideline 12.1) reflect the availability of different equipment and the expected higher degree of skill/experience required for paediatric advanced life support.
ANZCOR Guideline 12.2 is focused on paediatric advanced life support (PALS) ie. resuscitation with the aid of equipment and medications to restore and maintain airway, breathing and circulation in infants and children in a healthcare environment where cardiorespiratory arrest may be encountered. It applies to children and infants (excluding newborns ie. at time of birth). The guideline does not refer in detail to the resuscitation of the newborn which may be found in ANZCOR Guidelines 13.1 to 13.10.
Because equipment may not be immediately available in healthcare settings, PALS should be seen as the next stage in the continuum from BLS or PBLS (resuscitation without equipment and medications). Further details of PBLS for infants and children may be found in ANZCOR Guideline 12.1.
Terminology

Definitions of ‘newborn’, ‘infant’ and ‘child’ are based on combinations of physiology, age and physical size which influence the efficacy and practicality of performing resuscitative techniques.
For the purposes of paediatric life support guidelines, paediatric patients include infants (0 to 12 months of age ie. up to the first birthday), and children (up to the 18th birthday), excluding newborns. The term ‘newborn’ refers to an infant at the time of birth.
From a practical perspective, if the rescuer believes that the patient is a child, then they should follow paediatric guidelines and adult guidelines should be used for anyone who appears to be an adult. If the patient turns out to be a young adult, it is unlikely that any harm will have been done as the paediatric pattern of cardiorespiratory arrest has been demonstrated to continue into early adulthood.7
The exact age at which paediatric techniques, particularly the compression-ventilation ratio, should replace those used for newborns is not certain, especially for small premature infants. Infants whose cardiorespiratory physiology is in transition from an intra-uterine environment at birth to several hours after birth (ie. newborns) should preferably be managed as per neonatal guidelines (Section 13). Infants aged more than a few hours beyond birth should be managed according to paediatric guidelines (with a compression-ventilation ratio of 15:2) in the pre-hospital, emergency department, paediatric inpatient and paediatric intensive care unit environments.6
Epidemiology of cardiorespiratory arrest in infants and children

Cardiorespiratory arrest may occur as the end result of a wide variety of conditions in infants and children. In the paediatric population, cardiorespiratory arrest is more often secondary to respiratory (eg. hypoxaemia secondary to respiratory infection, trauma, drowning or poisoning) or circulatory failure (eg. hypotension secondary to septicemia or trauma) rather than a primary arrest from an arrhythmia.
The initial cardiac rhythm discovered is usually (85%) asystole or PEA.8 The incidence of ventricular fibrillation or pulseless ventricular tachycardia as the initial rhythm is approximately 15%.8 Ventricular fibrillation may be present more commonly as the initial presenting rhythm in association with congenital heart conditions, poisoning with cardioactive medications and membrane ion channelopathies such as congenital long QT syndrome. When ventricular fibrillation is encountered later in the course of resuscitation, it is more likely to represent an agonal rhythm. Respiratory arrest may occur alone, but if not treated promptly, it may rapidly progress to cardiac arrest.
Preventing cardiorespiratory arrest: recognition of deterioration & paediatric rapid response systems

Cardiorespiratory arrest in children is usually preceded by a period of recognisable deteriorating respiratory or cardiovascular function (or both) and is therefore often predictable and may be preventable. If deterioration is recognised and treated early, cardiorespiratory arrest may be prevented. However, many barriers to recognition and treatment of a child with a deteriorating illness exist.9
Paediatric rapid response systems - medical emergency team (MET) or rapid response team (RRT) systems – may help to reduce the incidence of respiratory and/or cardiac arrest in hospitalised children (outside of the intensive care setting) and may reduce hospital mortality. An evidence update performed as part of the ILCOR 2020 process1 identified a number of additional studies addressing this topic but findings were not sufficiently different to change the ILCOR 2015 recommendation.2
ANZCOR suggests that paediatric rapid response systems should be implemented in all hospitals that care for children [CoSTR 2015, weak recommendation, very low quality of evidence].2
Triggers for activation of rapid response systems may include single-parameter or multi-parameter aggregate scores (early warning scores) and may incorporate activation for clinician or family concern. The ILCOR PLS Taskforce conducted a scoping review to determine if new evidence was available since the 2015 COSTR recommendation. The ILCOR scoping review identified 3 systematic reviews and one other scoping review published after 2015; all noted the limited evidence for the usefulness of the paediatric early warning scores (PEWS) for preventing physiologic deterioration and improving clinical outcomes but did support the use of bedside PEWS to decrease clinically important deterioration on the wards in non-tertiary care/community hospitals.1 It was concluded that the implementation of PEWS should be part of an overall clinical response system, with a higher value placed on improving healthcare provider ability to recognise and intervene for patients with deteriorating illness over the expense incurred by a healthcare system committing significant resources to implement PEWS.1
Recognising cardiorespiratory arrest

Cardiorespiratory arrest should be suspected in a child or infant who is unresponsive and not breathing normally. Additional signs include pallor, cyanosis and absence of pulse. Health professionals may use pulse palpation as a part of their assessment but if a pulse cannot be confidently identified within 10 seconds, or there is uncertainty, cardiopulmonary resuscitation (CPR) should be commenced.5 In two studies of paediatric cardiac arrest, healthcare personnel could not reliably determine the presence or absence of a pulse when other information about the presence or absence of a circulation was unknown to them.10,11 An evidence update was conducted as part of the ILCOR 2020 process1 but no studies were identified that would necessitate a change from the 2010 recommendation.5
ANZCOR suggests that palpation of a pulse (or its absence) is not reliable as the sole determinant of cardiac arrest and need for chest compressions. In infants and children who are unresponsive and not breathing normally, healthcare providers should begin CPR unless they can definitely feel a pulse within 10 seconds [CoSTR 2020].1
Sequence of actions in Paediatric ALS

Resuscitation should commence immediately with basic techniques in the healthcare setting and continue with the aid of medications and equipment as soon as these become available. Paediatric Advanced Life Support (PALS) may include the preservation of a patent airway by endotracheal intubation or other device, the provision of positive pressure ventilation via mechanical devices with oxygen, the treatment of cardiac arrhythmias, the treatment of the cause of cardiorespiratory arrest and the treatment of complications arising from its management.
A single rescuer encountering an unwitnessed collapse of an infant or child should shout for help then start CPR immediately. If help has not arrived within 1 minute, then the rescuer should go to get help. To minimise interruptions to CPR, it may be possible for the rescuer to carry the infant / small child with them while summoning help.
When more than one rescuer is available, one rescuer should start resuscitation while the other summons assistance.
A single first responder witnessing a sudden collapse (potential primary cardiac arrest) should prioritise obtaining help and then start CPR, as urgent defibrillation may be required [Good Practice Statement].5
When several rescuers are in attendance, the initial actions (eg. bag-valve-mask ventilation or ventilation via an advanced airway device, attaching monitoring equipment and access to the circulation) should be attempted simultaneously. Thereafter treatment should be guided by the cardiac rhythm.
Once the defibrillator arrives, the electrocardiograph (ECG) may be displayed using chest leads or defibrillator pads. The type of arrhythmia present will determine treatment choice of either medication therapy or immediate defibrillation while chest compressions and assisted ventilation (with oxygen) are continued.
Team-based approach to resuscitation

Although the sequence of actions in a resuscitation is usually described as a series of prioritised steps, PALS is a team activity and multiple urgent interventions will be performed safely and effectively in parallel. Paediatric resuscitation teams should not only train in the knowledge and skills for PALS but also in the teamwork and coordination aspects of efficient PALS interventions.
Airway & oxygen therapy

Optimising airway patency and provision of effective ventilation and oxygenation are central components in the management of critically ill or injured children.
Oxygen therapy

Both hypoxaemia and hyperoxaemia have been shown to have harmful effects and should be avoided. Studies involving newborns have demonstrated advantages of using room air (rather than oxygen) during resuscitation but this has not been shown for infants and older children.
The ILCOR 2020 PLS Taskforce conducted a scoping review to determine if new evidence was available to support a specific inspired oxygen concentration to use during attempted resuscitation of infants and children. The ILCOR scoping review1 identified no new human studies in infants (beyond the neonatal period) and children regarding oxygen concentration or its titration during cardiopulmonary resuscitation. The lack of human studies in infants or children that addressed the topic, and the indirectness of results from animal models were considered insufficient to alter the existing 2010 recommendations.5
As there is currently insufficient information to recommend a specific inspired oxygen concentration for ventilation during attempted resuscitation after cardiac arrest in infants and children, ANZCOR suggests that the highest concentration of oxygen available (100%) should be administered during initial resuscitation regardless of any preceding condition. Once ROSC is obtained and the child is stabilised, the concentration of oxygen delivered should then be titrated (Refer to ANZCOR Guideline 12.5)
In children who are breathing spontaneously, oxygen delivery may be supplemented by using nasal cannulae or appropriately sized oxygen face masks (preferably with a reservoir). In apnoeic patients, ventilation should be established using either a self-inflating bag-valve-mask (BVM) device or a modified Ayre’s t-piece circuit (connected to oxygen).
Self-inflating BVM devices should not be used to deliver oxygen to the child who is breathing spontaneously because minimal and unreliable amounts of oxygen are released passively from the exit valve.12
Airway positioning & manoeuvres

Airway opening manoeuvres including backward head tilt with chin lift [jaw support] (Figures 1 & 2) or jaw thrust (Figures 3 & 4) may be used to optimise the position of the infant’s (neutral) or child’s (‘sniffing position’ ie. mild extension of head on neck and mild flexion of neck on shoulders) airway. Hyperextension of the neck should be avoided as it may cause airway obstruction, especially in small infants. If a neck injury is suspected, only jaw thrust should be used [Good Practice Statement] to avoid worsening the injury.
The patency of the airway should be assessed by observation of movement of the chest and abdomen during breathing. An indrawing of the chest wall and/or distension of the abdomen with each inspiratory effort without expiration of air implies an obstructed airway.
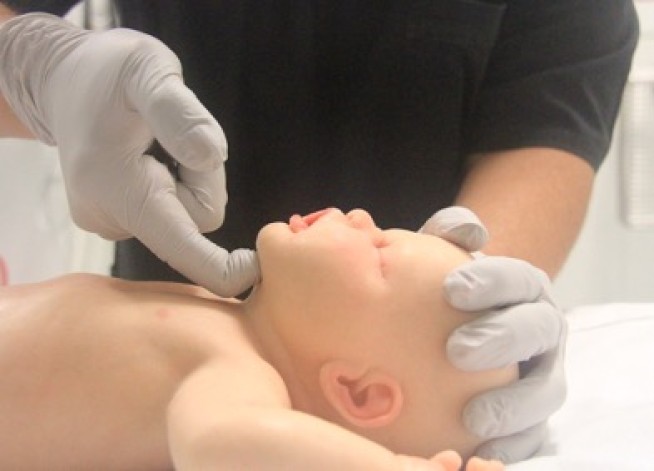
Figure 1: Head tilt with chin lift in an infant (neutral position)
[Image courtesy of Children’s Health Queensland, licensed under CC BY]
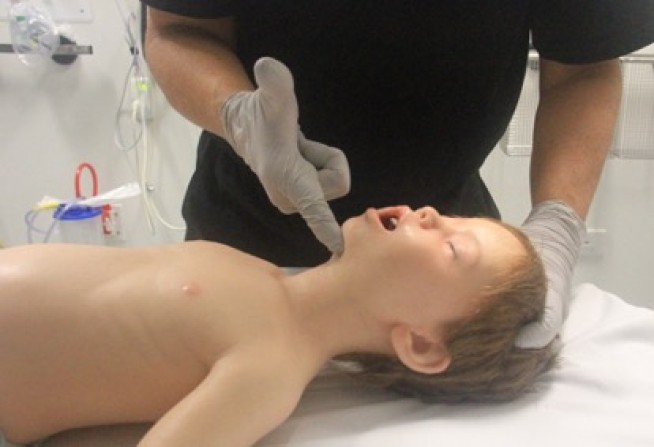
Figure 2: Head tilt with chin lift in a child (sniffing position)
[Image courtesy of Children’s Health Queensland, licensed under CC BY]
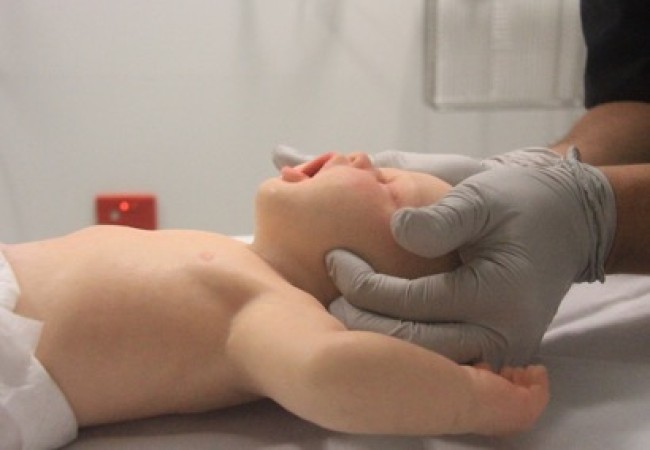
Figure 3: Jaw thrust in an infant
[Image courtesy of Children’s Health Queensland, licensed under CC BY]
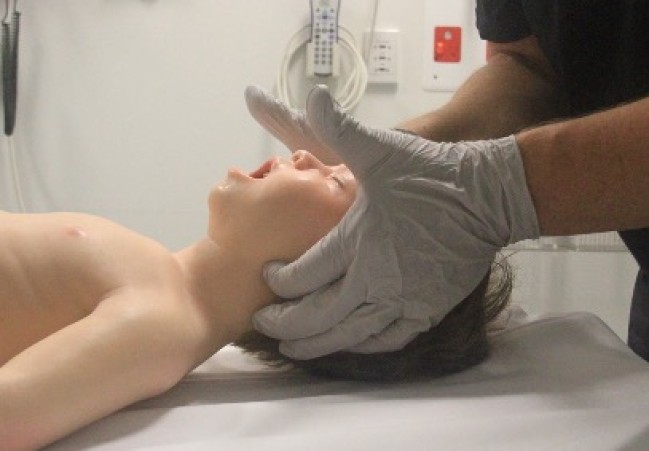
Figure 4: Jaw thrust in a child
[Image courtesy of Children’s Health Queensland, licensed under CC BY]
Airway clearance

If airway obstruction is not relieved by backward head tilt with chin lift or by jaw thrust, the pharynx should be inspected with the aid of a tongue depressor or laryngoscope and cleared of any secretions, vomitus or blood using a pharyngeal suction device (eg. Yankauer). Magill forceps may be used to extract a foreign body.
Airway adjuncts

Establishment and maintenance of an airway may be facilitated with an oropharyngeal or nasopharyngeal airway. It is important to note that these adjuncts do not protect the airway from aspiration of blood or secretions.
An oropharyngeal (Guedel) airway may help to open the airway in an unconscious child who has no gag reflex. Nasopharyngeal airways are usually better tolerated in conscious or semi-conscious children (with an effective gag reflex) but should be avoided in the setting of possible base of skull fracture or coagulopathy.
Initial appropriate sizes of devices may be estimated by placing the airways concave surface down along the face and using the following guidance:
- Oropharyngeal airway size is measured as the approximate distance from the centre of the mouth to the angle of the mandible (jaw) (Figure 5).
- Nasopharyngeal airway size is measured as the approximate distance from the tip of the nose to the tragus of the ear (Figure 6).
The diameter of a nasopharyngeal airway should approximate that of an endotracheal tube suitable for the child’s age.
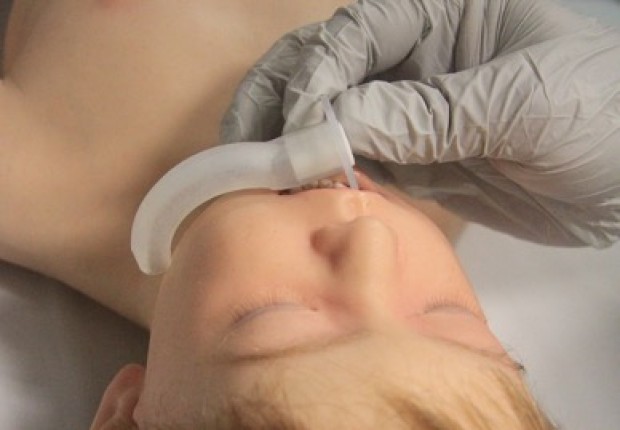
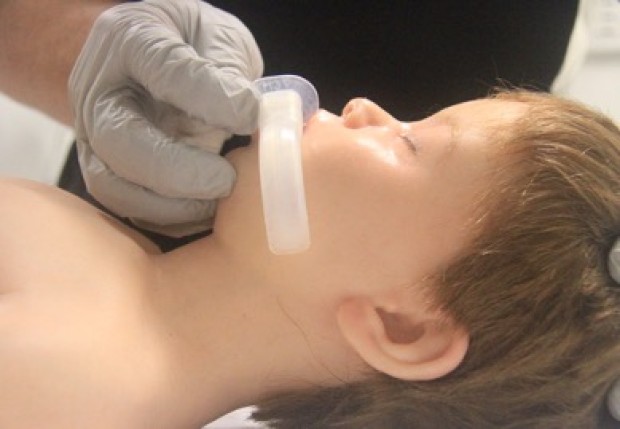
Figure 5: Sizing of oro-pharyngeal tube
[Images courtesy of Children’s Health Queensland, licensed under CC BY]
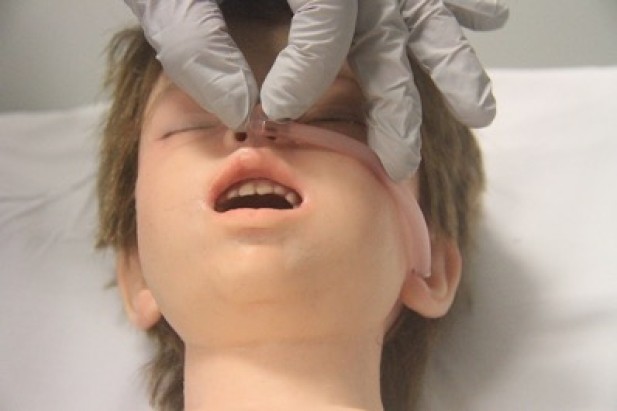
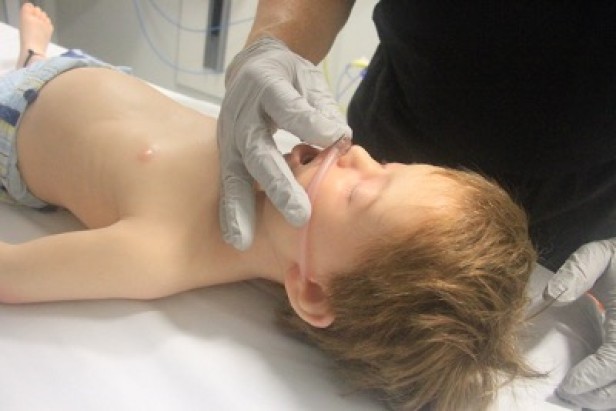
Figure 6: Sizing of naso-pharyngeal tube
[Images courtesy of Children’s Health Queensland, licensed under CC BY]
Facemasks

A range of mask sizes should be available to administer oxygen and/or ventilation.
The correctly sized facemask extends from the bridge of the nose to the space between the lower lip and point of the chin. Masks with inflatable or cushioned rims are preferable as they facilitate achievement of an airtight seal between the mask and the face.
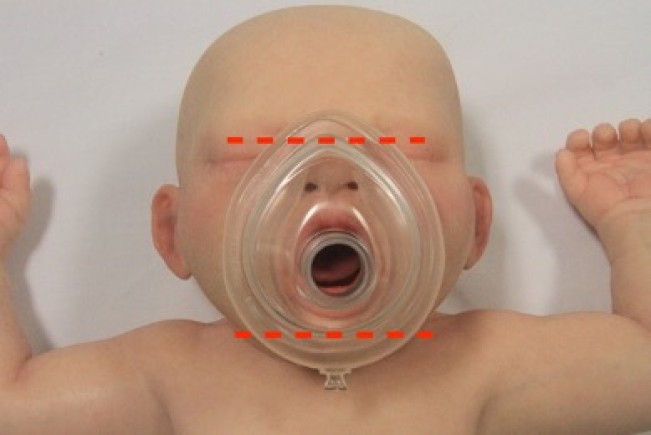
Figure 7: Appropriate mask size in a child
[Image courtesy of Children’s Health Queensland, licensed under CC BY]
Breathing

Ventilations in paediatric resuscitation

If spontaneous ventilation is not immediately resumed after airway repositioning, ventilation should be commenced using mouth-to-mouth ventilation or via a BVM, supraglottic airway (SGA) or endotracheal tube (ETT) depending on the training and expertise of the rescuers and the equipment available. Mechanical ventilation may be delivered by an oxygen inflated ventilation circuit (eg. Ayre’s T-piece circuit), self-inflating bag or an operator powered resuscitator dependent on an oxygen supply. The inspiratory time should be approximately one second.
The ILCOR 2020 BLS Taskforce conducted a systematic review1 examining the initial sequence of CPR in adults and children with OHCA: Compressions-Airway-Breaths versus Airway-Breaths-Compressions. The 2020 PLS taskforce scoping review1 did not identify any new human pediatric evidence regarding sequencing for initiating CPR published following the 2015 pediatric CoSTR.4
ANZCOR suggests that two initial ventilations should be provided before commencement of external cardiac compressions because asphyxial causes are more common than cardiac causes in paediatric cardiorespiratory arrest [Good Practice Statement]. In circumstances where the usual equipment used to provide ventilations (eg. BVM) is not immediately available, CPR should be commenced immediately with chest compressions.4
In 2017, a systematic review13 and an ILCOR Paediatric CoSTR14 were published on the topic of chest compression only compared with conventional CPR for infants and children. These included data from cardiac arrest registries in the USA and Japan and examined the effect of different bystander CPR methods - conventional CPR (including ventilation) versus compression only CPR versus no CPR – on survival and neurological outcomes. The results were supportive of the unchanged recommendation below.
ANZCOR continues to suggest that rescuers provide ventilations and chest compressions for paediatric IHCA and OHCA [CoSTR 2020, weak recommendation, very low quality evidence]. If rescuers are unable or unwilling to provide ventilations, they should at least perform chest compressions [CoSTR 2020, Good Practice Statement].
Advanced airway interventions in paediatric cardiac arrest

The management of the airway is central in paediatric resuscitation, particularly because respiratory conditions are a frequent cause of paediatric cardiac arrest. Positive pressure ventilation during cardiorespiratory arrest may be given by either BVM ventilation, supraglottic airway device or by endotracheal tube depending on the training and expertise of the rescuers. Placement of an advanced airway device such as a supraglottic airway (SGA) or endotracheal tube (ETT) may allow more effective resuscitation than ventilation using a BVM device. However, uncertainties remain about the risk and benefit of each method of managing the airway during CPR.
Associated challenges that may affect the quality of CPR provided include:
- provision of effective (but not excessive) ventilation
- delivery of continuous chest compressions
- the procedure resulting in prolonged interruptions in chest compressions
- risks of failed intubation attempts and unrecognised oesophageal intubation
As a part of the ILCOR 2019 CoSTR process, the Paediatric Taskforce performed a systematic review to identify and analyse all published evidence reporting outcomes of advanced airway placement during CPR in infants and children during OHCA and IHCA.15
As a result of inherent limitations in their design and data sources, the available studies provide only very-low-certainty evidence about whether attempting advanced airway placement during resuscitation (ie. before ROSC) improves resuscitation outcomes. The best available data show no benefit from advanced airway interventions, and some suggested association with harm for the critical outcomes of survival with favorable neurological outcome and survival to hospital discharge. The effects of placement of an advanced airway are uncertain for the short-term resuscitation outcomes of survival to hospital admission and ROSC.
Effective BVM ventilation and insertion of ETT or SGA are all difficult skills that require good initial training, retraining, and quality control to be performed consistently, safely, and effectively.
The benefit or harm associated with advanced airway–based resuscitation may differ across settings and available data does not help us to decide whether better outcomes might be achieved by advanced airway–based strategies by highly trained and experienced airway operators, during long distance transport, or in prolonged resuscitation situations. The analysed data are also relevant only to advanced airway interventions during CPR and do not pertain to airway management after ROSC or in other critical situations.
ANZCOR suggests the use of BVM ventilation rather than ETT or SGA insertion in the management of children during cardiac arrest in the out-of-hospital setting [weak recommendation, very low certainty of evidence]. There is insufficient evidence to support any recommendation about the use of ETT or SGA in the management of children with cardiac arrest in the in-hospital setting.15
Bag-Valve-Mask (BVM) Ventilation

Adequate inflation of the lungs is usually achievable with BVM ventilation but this can be a difficult technique for the non-expert. BVM ventilation is an acceptable technique during CPR as long as the lungs can be inflated adequately.
BVM may be given by either self-inflating resuscitation bags or oxygen flow inflating (exemplified by Jackson-Rees modified Ayre's T-piece) bags. Self-inflating resuscitation bags are recommended for the occasional resuscitator because of ease of operation. High flow oxygen should be added.
Supraglottic airway (SGA)

A supraglottic airway (SGA) may be used to establish an airway and give ventilation instead of using a BVM by persons trained in their use. They should not be used in semi-conscious patients or when the gag reflex is present. They are subject to dislodgment during transport. Their use should not replace mastery of BVM ventilation. The SGA is a suitable means of providing ventilation in situations where BVM ventilation has failed or is inadequate and ETT intubation is not possible.
Supraglottic airway sizes to suit body weight of newborns, infants and children differ according to the SGA brand and type so manufacturer’s guidelines should be followed. It should be noted that SGA size may be better estimated using ideal rather than actual body weight.
Endotracheal Intubation

Intubation of the trachea has several advantages but should not be attempted at the disadvantage of prolonging hypoxaemia [Good Practice Statement]. If intubation cannot be accomplished easily, oxygenation should be re-established by assisted or controlled positive pressure ventilation with a BVM device before a re-attempt at intubation. Endotracheal intubation offers several advantages, as it:
- establishes and maintains a patent airway,
- facilitates initial mechanical ventilation with 100% oxygen (and later accurate administration of lesser amounts),
- minimises pulmonary aspiration and pulmonary oedema,
- enables suctioning of the trachea,
- may be more practicable for airway maintenance and ventilation than BVM ventilation during prolonged management or transport.
This technique may be preferred in the hospital setting for maintenance of the airway and provision of mechanical ventilation after initial ventilation with BVM ventilation or SGA ventilation and to reduce the risk of aspiration.
The ILCOR 2020 PLS Task Force commissioned an evidence update comparing cuffed with uncuffed tracheal tubes1 to identify any evidence on the topic published since the last review of this topic in 2010.5 The evidence update identified 3 systematic reviews, 2 randomised controlled trials and 3 observational studies published since the previous review. The PLS task force agreed that the evidence identified supports the consideration of a systematic review about the use of cuffed versus uncuffed tubes in cardiopulmonary resuscitation to ascertain if the treatment recommendation requires modification. Until the completion and analysis of a new systematic review, the 2010 treatment recommendation remains in effect.
ANZCOR suggests that both cuffed and uncuffed tracheal tubes are acceptable for use in infants and children undergoing emergency intubation. If tracheal tubes are used, avoid excessive cuff pressures.5
For uncuffed tubes:
|
Age of child |
ETT size internal diameter (mm) |
|
Term newborn (2000-3000g) |
3.0 |
|
Term newborn (>3kg) |
3.0 – 3.5 |
|
Infant up to 6 months |
3.5 |
|
Infant 7-12 months |
4.0 |
|
Child >1 year |
Age (in years)/4 + 4 |
For cuffed tubes:
|
Age of child |
ETT size internal diameter (mm) |
|
Term newborn (>3kg) |
3.0 |
|
Infant up to 12 months |
3.0 |
|
Child 1-2 years |
3.5 |
|
Child >2 years |
Age (in years)/4 + 3.5 |
If insertion of a cuffed tube meets tracheal resistance, a tube 0.5 mm smaller should be used. If there is no leak around a tube with its cuff deflated, a 0.5 mm smaller tube should be inserted when the patient’s condition is stable.4
Irrespective of formulae, the correct size should enable adequate lung inflation with escape of a small volume of gas around the tube on application of moderate pressure. However, cuffed tubes or closer fitting uncuffed tubes may be preferable when lung compliance is poor. Initial insertion of a cuffed tube obviates the need to change a tube when oxygenation is compromised by a leak around a tube which is too small.
The tube should be inserted to a specified length to avoid accidental extubation or endobronchial intubation. The approximate depth of insertion measured from the centre of the teeth (or gums) for oral or nasal tubes are displayed in the following table:
|
Age of child |
Oral ETT Approx. insertion depth (cm) |
Nasal ETT Approx. insertion depth (cm) |
|
Term newborn |
9.0 |
11.0 |
|
Infant 6 months |
11.5 |
13.0 |
|
Child 1 years |
12.0 |
14.0 |
|
Child >1 year |
Age (in years)/2 + 12 |
Age (in years)/2 + 15 |
Although a guide, assessment of depth of intubation is not reliable during laryngoscopy because this is performed with the neck extended whereas on removal of the laryngoscope, the head assumes a position of neutrality or flexion thereby increasing depth of insertion. Initial intubation by the nasal route should not be attempted unless the oral route is obstructed.
Intubation by the oral route is invariably quicker, less likely to cause trauma and haemorrhage and the tube is more readily exchanged if the first choice is inappropriate. However, orally placed tubes are more likely than nasally placed tubes to dislodge or intubate a bronchus. The ETT should be secured to the face with adhesive tape.
Confirmation of correct placement should be undertaken immediately after insertion and frequently thereafter. In emergency conditions, the oesophagus or a bronchus may be mistakenly intubated. Moreover, displacement during resuscitation or transport may occur.
Successful endotracheal intubation may be indicated by:
- the tip of the tube is visualised passing through the vocal cords at intubation.
- bilateral lung inflation on auscultation of breath sounds in the axillae
- observation of intermittent rise and fall of the chest observed with each ventilation
- return and maintenance of oxygenation.
- exhaled CO2 detection (colorimetric detector or capnography)
The ILCOR 2020 PLS Task Force conducted an evidence update1 to determine if there was new evidence to support the use of devices to confirm advanced airway placement published after the most recent review of the topic in 2005.6 The evidence update identified one systematic review, 3 randomised controlled trials and relevant output from national surveys since the previous review. The PLS task force agreed that there is sufficient new evidence to suggest the need for a systematic review. Until the completion and analysis of a new systematic review, the 2005 treatment recommendation remains in effect.
ANZCOR suggests that confirmation of tracheal tube position using exhaled CO2 detection (colorimetric detector or capnography) should be used for intubated infants and children with a perfusing cardiac rhythm in all settings (eg. out-of-hospital, emergency department, intensive care unit, inpatient, operating room). In infants and children with a perfusing rhythm, it may be beneficial to monitor continuous capnography or frequent intermittent detection of exhaled CO2 during out-of-hospital and intrahospital or interhospital transport.6
Note that CO2 excretion cannot occur without pulmonary blood flow. Lack of CO2 detection implies non-tracheal intubation or lack of pulmonary blood flow, possibly due to excessive ventilation or inadequate chest compression or a combination of these factors. The position of the tube in the trachea should be re-checked immediately.
After intubation, an orogastric or nasogastric tube should be inserted to decompress the stomach which is often inflated by mask delivered positive pressure ventilation. An over-inflated stomach may impede the ability to oxygenate and ventilate, especially in infants, and also increases the risk of vomiting.
Use of cricoid pressure during intubation

The ILCOR 2020 PLS Task Force commissioned an evidence update about the use of cricoid pressure during endotracheal intubation1 to identify any evidence on the topic published since the last review in 2010.5 The evidence update identified 2 observational studies suggesting an association between external laryngeal manipulation, such as cricoid pressure, and increased difficulty during tracheal intubation of children in the emergency setting. The PLS Task Force concluded that they should consider the need for a comprehensive systematic review to determine if the existing recommendation should be amended. Until a new systematic review is completed and analysed, the 2010 treatment recommendation remains in effect.
ANZCOR suggests that, if cricoid pressure is used during emergency intubation in infants and children, it should be discontinued if it impedes ventilation or interferes with the speed or ease of intubation.
Ventilation rate with advanced airway during cardiac arrest

The ILCOR 2020 PLS Task Force conducted an evidence update1 to determine if there was new evidence to support optimal minute ventilation (product of tidal volume and respiratory rate/min) after the placement of an advanced airway during CPR in infants or children. The minute ventilation recommended in the 2010 CoSTR was based on expert consensus.5
There was no new evidence identified to support any specific ventilation rate for the infant or child with inadequate ventilation and a perfusing rhythm. The evidence update did identify a small single-center observational paper that reported an association of ventilation rates higher than 12 to 20/min with improved outcomes.16 Ongoing studies are anticipated to conclude later in 2020 that may provide further data.
ANZCOR suggests that, after placement of a secure airway, avoid hyperventilation of infants and children during resuscitation from cardiac arrest, whether asphyxial or arrhythmic in origin.1 A reduction in minute ventilation to less than baseline for age is reasonable to provide sufficient ventilation to maintain adequate ventilation-to-perfusion ratio during CPR while avoiding the harmful effects of hyperventilation. There are insufficient data to identify the optimal tidal volume or respiratory rate [CoSTR 2020, Good Practice Statement].
Circulation

The circulation may be assessed by looking movement, coughing or normal breathing.
Agonal respiration (gasping laboured breathing) describes an abnormal pattern of breathing (brainstem reflex) associated with cardiac arrest that should not be confused with normal breathing.
Healthcare providers may assess for a central pulse (carotid, brachial or femoral) but this should not delay CPR for more than 10 seconds. Palpation of a pulse (or its absence) is not reliable as the sole determinant of cardiac arrest and need for chest compressions.
Chest compression should be commenced if:
- the infant/child is unresponsive and not breathing normally or
- a pulse is not palpable or cannot be identified within 10 seconds, or the pulse rate is less than 60 beats per minute accompanied by signs of poor circulation.
Pulse check accuracy was the subject of an ILCOR 2020 PLS Taskforce evidence update.1 There was insufficient new evidence found to generate a change from the 2010 recommendation.
ANZCOR suggests that, in infants and children who are unresponsive and not breathing normally, healthcare providers should begin CPR unless they can definitely palpate a pulse within 10 seconds.1
To give chest compressions, the child should be placed on a firm surface and compression directed to the lower sternum.
Compression depth & release

A scoping review was conducted as part of the 2020 ILCOR PLS Taskforce process1 to identify new evidence since the 2015 guidelines regarding paediatric chest compression depth. No new published evidence was identified with this scoping review but the PLS Task Force did identify an ongoing large prospective observational international multicentre study on CPR quality using dual-sensor CPR feedback devices: the pediRES-Q study.17 While awaiting results of this study, treatment recommendations are unchanged from 2015.4
ANZCOR suggests that rescuers compress the chest by at least one third the anteroposterior dimension, or approximately 4cm in an infant and 5cm in a child [CoSTR 2015, weak recommendation, very low-quality evidence].
Method of compression

An evidence update was performed by the ILCOR 2020 PLS Taskforce1 to identify available evidence about different techniques for chest compressions of infants and children since the previous review was published in 2010. The evidence update identified several studies published after 2010, and the task force agreed that these studies suggest the need to consider conducting a systematic review. Until a new systematic review is completed and analysed by the PLS TaskForce, the 2010 treatment recommendation remains in effect.
- Infant: Chest compression for an infant can be performed with the two-thumb technique or two-finger technique [LOE IV]. The two-thumb technique (Figure 8) is the strongly preferred technique for healthcare rescuers5 [Good Practice Statement]. With this technique, the rescuer’s hands encircle the chest and the thumbs compress the sternum. Care should be taken to avoid restricting chest expansion during inspiration. The two-finger technique (Figure 9) may be preferred by a single rescuer to minimise the transition time between chest compression and ventilation [Good Practice Statement].
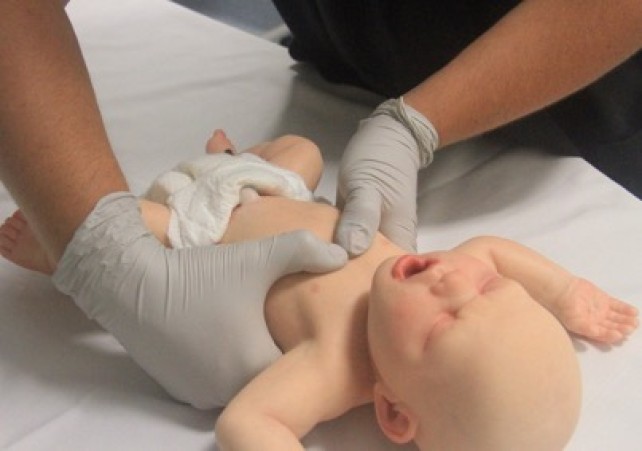
Figure 8: Two-thumb compression technique in an infant
[Image courtesy of Children’s Health Queensland, licensed under CC BY]
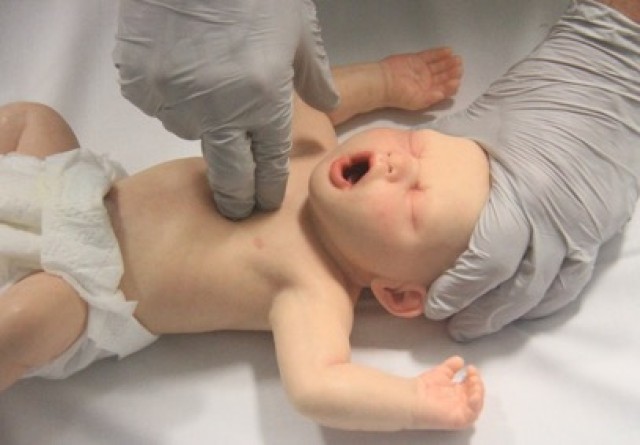
Figure 9: Two-finger compression technique in an infant
[Image courtesy of Children’s Health Queensland, licensed under CC BY]
- Child: Chest compression can be performed with the ‘heel’ of one hand (Figure 10) or the two-handed technique (Figure 11) to achieve a compression depth of at least one third of the anterior-posterior (AP) diameter of the chest [Good Practice Statement].
There is insufficient evidence to make a recommendation for or against the need for a circumferential squeeze of the chest when performing the 2 thumb-encircling hands technique of external chest compression for infants.1 Whatever technique is employed, pressure over the ribs and abdominal viscera should be avoided.
Approximately 50% of a compression cycle should be devoted to compression of the chest and 50% to relaxation to enable full recoil of the chest wall.
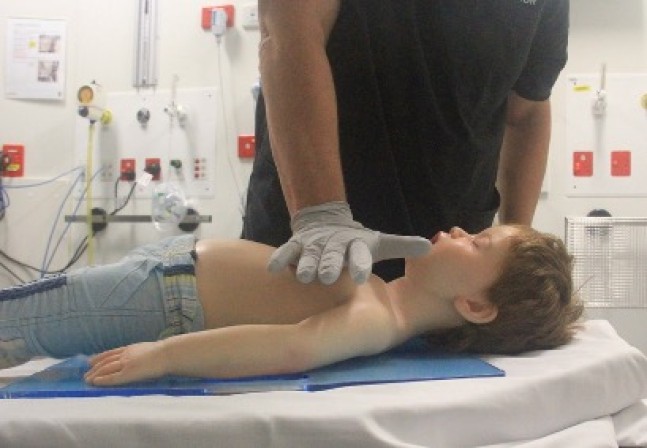
Figure 10: One-handed compression technique in a child
[Image courtesy of Children’s Health Queensland, licensed under CC BY]
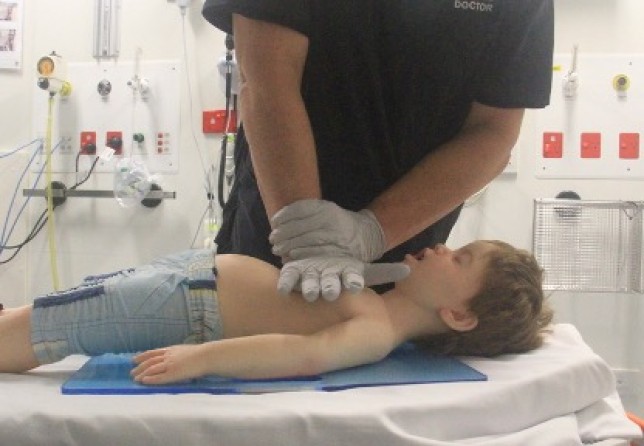
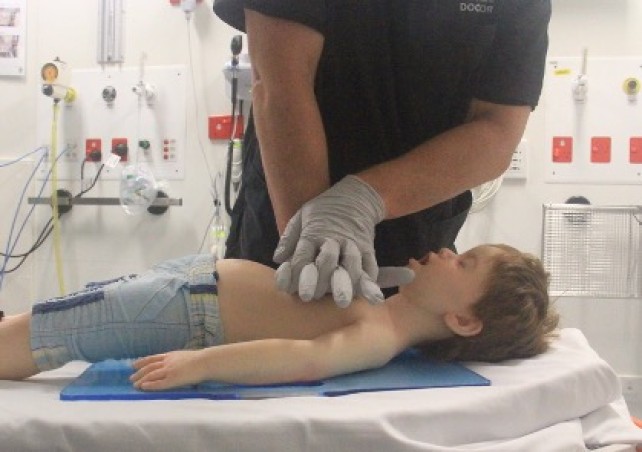
Figure 11: Two-handed compression techniques in a child
[Images courtesy of Children’s Health Queensland, licensed under CC BY]
Ratios and rates of compressions and ventilations1

Rescuers trained in PALS should use a compression-ventilation ratio of 15:2 for each duty cycle. There should be pauses for ventilation (BVM ventilation, SGA ventilation) to allow adequate expansion of the lungs.
Ventilation should ideally be timed to occur just after a compression. This will minimise (but not eliminate) simultaneous ventilation and chest compression. To minimise the pause for lung inflation, chest compression should be recommenced during the expiratory phase of the second inflation. Delivering 5 cycles per minute in this way will yield approximately 75 to 90 compressions and 10 breaths per minute.
When the airway is secured with an endotracheal tube (or tracheostomy), chest compressions should be continuous (uninterrupted by ventilation) at a rate of 100 to 120 compressions per minute. Ventilation should be given at a rate as discussed in Section 9.7. Care should be taken to avoid hyperventilation since this may compromise the effectiveness of external cardiac compression and the resultant hypocapnia may also cause cerebral vasoconstriction and decrease cerebral perfusion. During uninterrupted chest compressions, ventilation should be delivered during the release phase of a compression.
Peripheral venous access

Any pre-existing functioning venous line can be used provided it does not contain any medication or electrolyte which caused the cardiorespiratory arrest.
Peripheral veins may be found on the dorsum of the hand, wrist, forearm, cubital fossa, foot and ankle (long saphenous). The external jugular is often distended during CPR but cannulation is impeded by performance of endotracheal intubation. Cannulation of the external jugular is facilitated when the patient is intubated and the head is turned to the opposite side. Cannulation of the femoral vein is an option facilitated by use of ultrasound.
Intraosseous injection and infusion

The intraosseus (IO) route offers safe and ready access to the circulation. The bone marrow has a rich blood supply and injected medications are distributed as fast and attain the same plasma concentrations as those injected intravenously. All resuscitative medications and fluids may be given via the IV or IO route.5 Although most commonly used for young children, the IO route can be used for patients of any age including premature newborns and adults. Establishment of IO access is quicker to achieve than IV access in severely dehydrated children and fluids administered by this route stabilise vital signs as quickly as fluids given intravenously. Any IV fluid or medication may be administered with the aid of gravity, infused under pressure or injected from a syringe. Although many sites may be used, the antero-medial surface of the proximal or distal tibia are the most suitable puncture sites during resuscitation of infants and children.
Intraosseous needles, bone marrow injection guns and drills are specifically manufactured for this purpose. The needle is inserted perpendicularly to the bone surface. If a hand-held needle is used, a rotary action is used to traverse the cortex. A loss of resistance signals entry to the marrow space.
Correct positioning of the IO needle, confirmed by aspiration of bone marrow or injection of 0.9% NaCl without extravasation, is necessary to avoid compartment syndrome. Bone marrow may be used reliably for venous biochemical and haematological analysis but not for venous blood gas tensions. Contraindications to IO needle insertion include local trauma, infection and bone disorders.
IO medication administration was the subject of a systematic review conducted as part of the ILCOR 2020 process.1 No new paediatric studies were identified since the recommendation in 2010,5 so our advice remains unchanged.
ANZCOR suggests that, in the setting of cardiac arrest, the intraosseous route is recommended if peripheral or central venous access is not already in place.5
Central venous cannulation

If a central venous line is already in place, it should be used in preference to any other route, but central venous cannulation via the subclavian or internal jugular veins during CPR is not recommended as it wastes time and may be hazardous in this circumstance [Good Practice Statement].
Fluid and medications

Fluids and medications are used in resuscitation to support cardiovascular physiology and organ perfusion and to ameliorate underlying pathophysiologic processes to reduce morbidity and mortality. Topics that were evaluated as part of the ILCOR 2020 CoSTR process included the optimal ways to calculate body weight for prescribing medications dosed by weight, amiodarone versus lidocaine for shock-resistant VF or pVT, and the role of sodium bicarbonate and of calcium in the management of cardiorespiratory arrest.1
All IV and IO medications should be flushed with small boluses of 0.9% NaCl or 5% glucose (for amiodarone). This ensures that the medications enter the circulation and prevents precipitation or inactivation, as occurs when sodium bicarbonate mixes with calcium or when sodium bicarbonate mixes with adrenaline (epinephrine).
Fluids and medication doses

An evidence update was performed by the ILCOR 2020 PLS Taskforce1 to identify available evidence about preferred methods for calculating paediatric medications doses since the previous review was published in 2010. The search performed for this evidence update identified multiple publications relating to paediatric weight estimation, considering many different methods of weight estimation. In light of the volume of paediatric publications identified, the taskforce agreed that there is sufficient evidence to warrant conducting a systematic review. Until the systematic review is completed and analysed by the PLS Taskforce, the 2010 treatment recommendation remains in effect.
ANZCOR suggests the following in relation to calculation of medication and fluid doses in paediatric resuscitation:
- To calculate the dose of resuscitation medications, use the child’s weight if known. If the child’s weight is unknown, it is reasonable to use a body length tape with precalculated doses.
- In non-obese paediatric patients, initial resuscitation medication doses should be based on actual body weight (which closely approximates ideal body weight). If necessary, body weight can be estimated from body length.
- In obese patients, the initial doses of resuscitation medications should be based on ideal body weight that can be estimated from length. Administration of medication doses based on actual body weight in obese patients may result in medication toxicity.
- Subsequent doses of resuscitation medications in both non-obese and obese patients should take into account the observed clinical effects and toxicities. It is reasonable to titrate the dose to the desired therapeutic effect, but it should not exceed the adult dose.
Fluid therapy

If hypovolaemia is suspected as the cause of cardiorespiratory arrest, intravenous or intraosseous crystalloid may be used initially for resuscitation4 as a bolus of 10 to 20mL/kg. Additional boluses or colloid solution should be titrated against the response.
Adrenaline (epinephrine)

Both alpha and beta effects of adrenaline (epinephrine) are useful in management of cardiorespiratory arrest. Alpha vasoconstrictor effects diverts blood to the cerebral and coronary circulation and can facilitate defibrillation while beta effects are chronotropic and inotropic.
Adrenaline (epinephrine) is used to treat asystole, severe bradycardia, VF, pulseless VT and pulseless electrical activity (PEA). The initial and any subsequent dose by the IV or IO route is 10 micrograms/kg with a maximum single dose of 1mg. It should be given every second loop (ie. every 3 to 5 minutes) of the PALS pathway (see Section 7).
Adrenaline (epinephrine) administration for cardiac arrest was previously reviewed in the 2015 CoSTR.4 A systematic review was performed as part of the ILCOR 2020 PLS Taskforce process1 to identify available new evidence about the effectiveness and timing of adrenaline (epinephrine) administration. The systematic review identified no paediatric randomised controlled trials (RCTs) on this topic but did identify one observational study of paediatric IHCA18 and 4 observational studies of OHCA19-22 comparing the administration of the initial dose of adrenaline (epinephrine) earlier or later than current guideline recommendations. There were also 2 observational studies on paediatric IHCA23,24 examining adrenaline (epinephrine) given more or less frequently than every 3-5 minutes after the initial dose. There were no identified observational studies of paediatric OHCA addressing the interval between adrenaline (epinephrine) doses.
In IHCA, for each of the critical outcomes (survival with good neurological outcome, survival to discharge, 24-hour survival, ROSC) there was very low-certainty evidence (downgraded for risk of bias) of benefit associated with earlier rather than later first adrenaline (epinephrine) dose (for each of before or after 15 minutes, 10 minutes, 5 minutes and 3 minutes after cardiac arrest).1
Similarly, for OHCA, for each of the critical outcomes (survival to discharge, 30-day survival, survival to intensive care, ROSC) there was very low-certainty evidence (downgraded for risk of bias) of benefit associated with earlier rather than later first adrenaline (epinephrine) dose (for each of before or after 15 minutes, 10 minutes, 5 minutes and 3 minutes after cardiac arrest).1
There were conflicting results in the observational studies regarding adrenaline (epinephrine) dose intervals.
ANZCOR suggests that the initial dose of adrenaline (epinephrine) in paediatric patients with non-shockable rhythm IHCA and OHCA be administered as early in the resuscitation as possible [weak recommendation, very low-certainty evidence].1
In the absence of consistent evidence regarding the optimal interval for subsequent adrenaline (epinephrine) doses in paediatric patients with IHCA or OHCA, ANZCOR suggests that the current recommended practice of administration at intervals of 3 to 5 minutes (or every second loop of the PALS pathway) continues [ANZCOR 2020, Good Practice Statement].
Amiodarone

Amiodarone is an antiarrhythmic medication with complex pharmacokinetics and pharmacodynamics. It may be used for shock-resistant ventricular fibrillation (VF) and pulseless ventricular tachycardia (pVT).4 The initial paediatric dose for shock-resistant VF and pVT is a bolus of 5 mg/kg (maximum dose 300mg). In children, amiodarone can be used to successfully treat a wide range of other tachyarrhythmias, notably atrial tachycardias, (recurrent) supraventricular tachycardia, pulsatile ventricular tachycardia, junctional ectopic tachycardia and wide QRS-complex tachycardia (Refer to ANZCOR Guideline 12.3).
Previous CoSTR statements evaluating the use of antiarrhythmic medications during paediatric VF/pVT cardiac arrest have included extrapolated evidence from adult OHCA studies and case series of children with life-threatening ventricular arrhythmias but not cardiac arrest. The ILCOR Pediatric Task Force agreed that the 2018 ILCOR CoSTR25 would not review evidence extrapolated from studies of adult cardiac arrest. A single observational cohort study with 302 patients was available for analysis. For the critical outcome of survival to hospital discharge, the study found no difference in effect for lidocaine (lignocaine) compared with amiodarone.25
ANZCOR suggests that amiodarone or lidocaine (lignocaine) may be used for the treatment of paediatric shock-resistant VF or pVT (weak recommendation, very low-quality evidence).1
Amiodarone has become the standard antiarrhythmic medication for use in paediatric shock resistant VF and pVT, and it is reasonable for this to continue in the absence of strong evidence to change practice (Good Practice Statement).
Calcium

Calcium may be used as an inotropic or vasopressor agent but it has no place in the management of an arrhythmia unless it is caused by hyperkalaemia, hypocalcaemia, hypermagnesaemia or calcium channel blocker.4
Calcium (0.15 mmol/kg) is the antidote to hypotension caused by a calcium channel blocker. The IV or IO dose is 0.2 mL/kg of 10% calcium chloride (calcium chloride dihydrate) or approximately 0.7 mL/kg (20 mg/kg) of 10% calcium gluconate (calcium gluconate monohydrate).
An evidence update was performed by the ILCOR 2020 PLS Taskforce1 to identify available updated evidence about use of calcium in paediatric arrest. Insufficient new evidence was found to consider a systematic review of this topic, so the recommendations of 2010 remain in effect.5
ANZCOR suggests that routine use of calcium for infants and children with cardiorespiratory arrest is not recommended in the absence of hypocalcemia, calcium channel blocker overdose, hypermagnesemia, or hyperkalemia.
Glucose

Hypoglycaemia may be present in paediatric critical illness, particularly in infants. Hyperglycaemia also occurs in paediatric critical illness and is associated with increased mortality but it is not known if this is the cause. Normal blood glucose level is 3 to 8 mmol/L.
The blood glucose level should be checked during CPR and after ROSC with the aim of ensuring normoglycaemia (Refer to ANZCOR Guideline 12.5). Hypoglycaemia may be treated with 2 mL/kg 10% glucose by rapid IV or IO infusion. Avoid extravasation, especially from peripheral veins, and avoid overdosage.
Lidocaine (lignocaine)

Although lidocaine (lignocaine) has a membrane stabilising effect and a potential to aid defibrillation, it may increase the defibrillation threshold. The dose of lidocaine (lignocaine) is 1 mg/kg IV or IO.
A comparison of lidocaine (lignocaine) and amiodarone was performed as part of the 2018 ILCOR CoSTR process (Refer to section 13.4 above on Amiodarone).
ANZCOR suggests that amiodarone or lidocaine (lignocaine) may be used for the treatment of paediatric shock-resistant VF or pVT (weak recommendation, very low-quality evidence).1
Magnesium

Hypomagnesaemia may cause life-threatening ventricular tachyarrhythmia, particularly when associated with hypokalaemia. Magnesium is the preferred antiarrythmic treatment for polymorphic ventricular tachycardia (Torsade de pointes – “Twisting of peaks”) due to acquired or congenital prolonged QT interval syndromes.6
The IV or IO bolus dose of magnesium (magnesium sulfate heptahydrate) is 0.1 to 0.2 mmol/kg followed by an infusion of 0.3 mmol/kg over 4 hours.
Potassium

Hypokalaemia may cause a life-threatening tachyarrhythmia. Emergency treatment is the IV or IO administration of KCl 0.03 to 0.07 mmol/kg by slow injection over several minutes. If the situation is critical but not immediately life-threatening, severe hypokalaemia may be treated with an infusion of 0.2 to 0.5 mmol/kg/hour to a maximum of 1 mmol/kg.
Extreme caution in the use of concentrated solutions of potassium is advised. Infusions should only be given by infusion pumps and frequent (every 30 to 60 minutes) serum monitoring with continuous ECG display is required, preferably in an intensive care unit setting. Mistakes in the calculation of potassium requirement and inadvertent administration of potassium may cause avoidable deaths. Therapies which rapidly decrease serum potassium level include:
- intravenous glucose PLUS insulin;
- inhaled or intravenous salbutamol PLUS intravenous glucose;
- a combination of these agents (insulin PLUS glucose PLUS salbutamol) with or without sodium bicarbonate;
- sodium bicarbonate alone (least effective).
Sodium bicarbonate

Sodium bicarbonate has a limited and unproven place in the management of paediatric cardiorespiratory arrest. Administration of IV or IO sodium bicarbonate neutralises hydrogen ions in the blood but in doing so produces carbon dioxide which may re-enter cells to exacerbate intracellular acidosis. Other deleterious effects include hypernatraemia and hyperosmolality which may depress myocardial function.
Administration of sodium bicarbonate may be considered in severe metabolic acidosis (pH < 7.1). The IV or IO dose is 0.5 to 1 mmol/kg after adequate ventilation with oxygen and chest compression have been established. The most effective treatment for acidemia in cardiac arrest is high quality CPR.
An evidence update was performed by the ILCOR 2020 PLS Taskforce1 to identify available updated evidence about use of bicarbonate in paediatric arrest. Insufficient new evidence was found to consider a systematic review of this topic, so the recommendations of 2010 remain in effect.5
ANZCOR suggests that administration of sodium bicarbonate is not used in the management of pediatric cardiac arrest. It has a specific role in hyperkalaemia and arrhythmias associated with tricyclic antidepressant overdose.
Defibrillation

Defibrillators may be either manual or automated (AED) and need to be able to deliver shocks in the range of 0.5 to 4 J/kg. Shocks may be delivered through either pads (preferred) or paddles.
Since defibrillators have stepped energy levels, the exact energy may not be available to conform to the dosage recommendations. In this case, the closest level to the dose should be selected. To deliver a shock, one electrode (self-adhesive pad) is placed over the cardiac apex (in the left mid-axilla opposite the xiphoid) and the other to the right of the upper sternum (antero-lateral position). Alternatively, pads may be placed in antero-posterior positions (one over the left of the lower sternum and the other below the left scapula). Pads should not be permitted to touch to avoid bridging and ineffective delivery.
The defibrillator should be charged while chest compressions are being carried out in order to minimise interruptions to CPR.
Care should be taken to ensure that no person is touching the patient at the time of discharge. If after charging, the need for defibrillation dissipates, the charge should be discharged (‘dumped’) safely.
Pad Size, Type and Placement for Paediatric Defibrillation

The topics of pad size and placement and adhesive pads compared with paddles were last reviewed in 2010.5 In the decade since the review, technological advances have been rapid, so an evidence update was performed as part of the ILCOR 2020 PLS CoSTR process.1 The evidence update did not identify sufficient evidence to suggest the need for a systematic review so the 2010 treatment recommendations for both topics remain in effect.
ANZCOR suggests that self-adhesive defibrillation pads be used in infants and children in cardiac arrest. The largest size pads that fit an infant’s or child’s chest without touching each other should be used.1
Pads allow chest compression to continue while charging, probably permit faster resumption of chest compression after delivery of a shock, may be safer and may allow easier use of an antero-posterior position which may be more efficacious than the standard antero-lateral positions of pads. Dextrocardia may be present with congenital heart disease and the position of the pads should be altered accordingly.
Energy doses for paediatric defibrillation

A scoping review was conducted as part of the ILCOR 2020 PLS CoSTR process.1 The review identified a single 2019 systematic review of pediatric human and animal studies.26 This systematic review identified no studies linking the initial or cumulative energy delivered to survival to hospital discharge and no link between long-term survival or survival with good neurological outcome.
The ideal energy dose for safe and effective paediatric defibrillation remains unknown but present evidence supports a dose of 2 to 4 J/kg.1 For the sake of simplicity, ANZCOR continues to suggest 4 J/kg for the initial dose of unsynchronised shock for VF and pVT, followed immediately by 2 minutes of CPR without waiting to analyse the rhythm [CoSTR 2015, weak recommendation, very-low-quality evidence].4
There is insufficient evidence from which to determine a dose for second and subsequent defibrillation energy doses.1 ANZCOR suggests a dose of 4J/kg (up to the recommended adult dose) for second and subsequent shocks.
Single or stacked shocks for paediatric defibrillation

An evidence update was performed by the ILCOR 2020 PLS Taskforce1 to identify available updated evidence in support of single compared with stacked shocks for pediatric defibrillation since the last review in 2010.5 There was no new evidence to suggest the need for a systematic review or to change the 2010 treatment recommendation.
ANZCOR suggests a single-shock strategy followed by immediate CPR (beginning with chest compressions) for children with OHCA or IHCA with VF or pVT.5
Three stacked shocks may be considered when the onset of a shockable rhythm is witnessed (with monitoring) in special circumstances such as:
- In the cardiac catheter laboratory
- In the intensive care unit or cardiac ward post cardiac surgery
- In other circumstances when a defibrillator is already attached.
Every effort should be made to ensure that interruption to CPR is minimal.
Automatic external defibrillation

Although a variable dose manual defibrillator is preferred, a semi-automated external defibrillator (AED) may be used for infants and children1 provided it is able to differentiate shockable from non-shockable rapid paediatric rhythms.
For children older than 8 years, a standard AED with adult pads and dose may be used.
In children 1 to 8 years, an AED with paediatric pads and/or paediatric dose attenuation is preferred. If that is not available, a standard AED with adult pads and dose may be used.
An evidence update was conducted by the ILCOR 2020 PLS Taskforce to determine if there were any published studies about the use of AEDs for infants with OHCA. The evidence update identified insufficient evidence to justify a systematic review or suggest the need for a change to the 2010 treatment recommendation and as a result, the 2010 treatment recommendation5 is unchanged.
For treatment of out-of-hospital VF/pVT in infants, the recommended method of shock delivery by device is listed in order of preference below. If there is any delay in the availability of the preferred device, the device that is available should be used. The AED algorithm should have demonstrated high specificity and sensitivity for detecting shockable rhythms in infants. The order of preference is as follows:
- Manual defibrillator
- AED with dose attenuator
- AED without dose attenuator
Management of non-shockable rhythms

Asystole or severe bradycardia

Asystole or pulseless severe bradycardia (less than 60 bpm) which is unresponsive to initial CPR should be treated with adrenaline (epinephrine) 10 mcg/kg (maximum dose 1 mg) via IV or IO routes.2 Possible underlying causes should be actively sought and treated.
If after adrenaline (epinephrine) a perfusing sinus rhythm cannot be restored, the priority of management is continuous high-quality CPR with repeated adrenaline (epinephrine) every 3 to 5 minutes (Refer to Section 12.3). Sodium bicarbonate 1 mmol/kg IV or IO may be considered in cases of prolonged arrest (Refer to Section 12.10).
Pulseless Electrical Activity (PEA)

Absent pulses despite relatively normal co-ordinated electrical activity visible on the electrocardiograph (ECG) is called pulseless electrical activity (PEA). PEA may be due to poor intrinsic myocardial contractility or it may be secondary to a number of remediable causes (the “4Hs and 4Ts”) including hypoxaemia, hypovolaemia, hypo/hyperthermia, hyperkalaemia, hypocalcaemia, severe acidosis, pericardial tamponade, tension pneumothorax, toxins or poisons or medications (including calcium channel blockers), or massive thrombotic or gaseous pulmonary embolism.
Management of PEA includes:
- continuous high-quality CPR
- administration of adrenaline (epinephrine) 10 mcg/kg (maximum dose 1 mg) IV or IO every 3 to 5 minutes (Refer to Section 12.3)
- treatment of possible underlying causes.
Since hypovolaemia or severe acidosis are possible treatable causes, persistent PEA may be treated with IV or IO boluses of colloid or crystalloid fluid 10 to 20 mL/kg (repeated as required after reassessment).
A chest radiograph, 12 lead ECG and echocardiograph (if available) may be obtained as they can help to detect underlying causes such as pericardial tamponade, pneumothorax, ventricular rupture or pulmonary embolism.
Management of shockable rhythms

Ventricular Fibrillation & pulseless Ventricular Tachycardia

Asynchronous multifocal ventricular contraction ie. ventricular fibrillation (VF) produces no cardiac output. Similarly, rapid wide-QRS complex ventricular tachycardia (VT) may produce no cardiac output. The only effective treatment is DC unsynchronised cardioversion (commonly referred to as “defibrillation”), which simultaneously depolarises all contractile tissue and may allow resumption of sinus rhythm. If the onset of VF or pVT is witnessed on an ECG monitor, such as in the ICU environment, defibrillation should be attempted before any other treatment.
The ideal energy dose for safe and effective paediatric defibrillation is unknown (Refer to Section 14.2) but for the sake of simplicity ANZCOR continues to suggest 4 J/kg for the initial and subsequent doses using a biphasic (preferable) or monophasic shock for VF and pVT, (CoSTR 2015, weak recommendation, very-low-quality evidence) followed immediately by 2 minutes of CPR without waiting to analyse the rhythm [CoSTR 2015, weak recommendation, very-low-quality evidence].4 Manual defibrillators are preferred in children. If a manual defibrillator is not available, it is appropriate to use an AED for children (Refer to Section 13.4).
If after defibrillation and a further 2 minutes of CPR, a perfusing sinus rhythm cannot be restored, the priority of management is:
- further single DC shocks (4 J/kg up to recommended adult dose) every 2 minutes
- continuous high-quality CPR in between shocks
- administration of adrenaline (epinephrine) 10 mcg/kg (maximum dose 1 mg) IV or IO every 3 to 5 minutes (Refer to Section 12.3) ie. after every second shock
- treatment of possible underlying causes.
Persistent or refractory VF or pVT may be treated with antiarrhythmics such as amiodarone 5 mg/kg (maximum dose 300 mg) IV or IO as a bolus (Refer to Section 12.4).
If amiodarone is unavailable as an anti-arrhythmic for DC-shock resistant VF or pVT, lidocaine (lignocaine) may be used as an alternative (Refer to Section 12.7) in a dose of 1 mg/kg IV or IO.
Witnessed onset of monitored VF/pVT

Three stacked shocks (4 J/kg, 4 J/kg, 4 J/kg) may be given rather than the standard single shock of 4 J/kg when the onset of a shockable rhythm is witnessed with monitoring in special circumstances (Refer to Section 13.3).
AEDs are not able to deliver stacked shocks.
Monitoring and improvement of quality outcomes in resuscitation

Physiological monitoring and feedback during CPR can facilitate the adjustment of CPR delivery during resuscitation and, as a result, may improve the quality of resuscitation and even resuscitation outcomes.1 Such monitoring may also allow for ‘individualised CPR’ tailored to the patient’s needs and their responses to resuscitation interventions.1
Vital signs

Routine monitoring of heart rate, respiratory rate and blood pressure are essential for infants and children with critical illness. It is prudent to have ready access to or have displayed the normal age-related values for rapid reference.
Oximetry

Pulse oximetry (SpO2) is essential monitoring in all critically ill patients. It equates well to arterial haemoglobin-oxygen saturation (SaO2) but not when the SaO2 is below 70%. The relationship between haemoglobin-oxygen saturation and partial pressure of oxygen in arterial blood (PaO2) is not linear. It should be noted that a SpO2 of 90%, although only 10% below normal haemoglobin-oxygen saturation, represents a partial pressure of oxygen in arterial blood (PaO2) of 60 mmHg which is 40 mmHg below normal.
End-tidal CO2

End-tidal carbon dioxide (ETCO2) monitoring has been recommended to confirm tracheal tube placement since ILCOR 2000.27 In addition, ETCO2 detection during positive pressure ventilation may guard against inadvertent extubation, particularly when the intubated patient undergoes transport to, within or between hospitals. Small movements of the head and neck, as may occur for example on transfer from one trolley to another or to a bed, may easily dislodge an endotracheal tube.
ETCO2 monitoring can also offer an indirect indication of cardiac output and pulmonary blood flow (noting caveats in relation to pulmonary blood flow and ventilation: perfusion ratio or with rapid changes caused by deterioration or response to effective treatment). As a result, ETCO2 has been proposed as a method to evaluate the effectiveness of CPR and to identify possible ROSC. A rapid increase in ETCO2 may be associated with improved CPR (or ROSC), and a sustained decline or persistently low ETCO2 may be observed in the absence of ROSC.
A scoping review was performed by the ILCOR 2020 PLS Taskforce1 to identify available evidence to support the use of ETCO2 to provide feedback to guide resuscitation efforts.
Evidence from the scoping review was insufficient to recommend consideration of a systematic review on the topic so the guidance from the ILCOR 2015 CoSTR5 is unchanged in that a recommendation is still too speculative.
Electrocardiograph (ECG)

The ECG should be displayed with either defibrillator electrodes or pads. Medication therapy or immediate direct current shock is administered according to the existing rhythm. Electrolyte status, especially that of potassium and calcium should be checked and may be indicated by ECG patterns.
Intra-arterial blood pressure monitoring during CPR

Maintenance of adequate arterial systolic (compression) and diastolic (relaxation) or mean pressure during CPR is crucial to maintain coronary and cerebral perfusion. Maintaining a sufficient minimum threshold blood pressure should be associated with improved clinical outcomes.1 It is unknown if CPR directed to meet individualised rather than uniform standard blood pressure targets will improve outcomes from cardiac arrest.
A scoping review was performed by the ILCOR 2020 PLS Taskforce1 to identify available evidence on this topic published after 2015.
There was no association between blood pressures measured during CPR and neurological outcomes in an observational study of survivors of pediatric critical care (including cardiac critical care).28 In an observational study of a highly selected pediatric critical care population with arterial pressure monitoring in place when cardiac arrest developed, there was a significant association between a mean diastolic blood pressure of 25 mmHg or greater in infants and 30 mmHg or greater in children within the first 10 minutes post-arrest and their survival as well as with survival with favourable neurological function.29
For children with IHCA and an arterial line already in place, hemodynamic-directed CPR may be considered but at present the confidence in effect estimates is so low that a recommendation is too speculative.
Point of Care Ultrasound (POCUS)

There is very limited paediatric evidence documenting the use of ultrasonography to identify reversible causes of arrest, for prognostication, or to determine cardiac futility.1 In the 2020 scoping review, the ILCOR PLS Taskforce warned against rapid implementation of POCUS into paediatric practice without sufficient evidence, despite its great potential and widespread acceptance. Acquisition and interpretation of images in children is more complex, especially in children with pre-existing heart disease. Furthermore, there are significant material and training costs which might be important in low-resource settings.
There is currently insufficient evidence to recommend for or against the routine use of bedside ultrasound and echocardiography during a paediatric arrest.1 ANZCOR suggests that ultrasonography may be considered to identify potentially treatable causes (4H4T) of an arrest when appropriately skilled personnel are available, but the benefits must be carefully weighed against the known deleterious consequences of interrupting chest compressions.
Near-infrared spectroscopy (NIRS)

Near-infrared spectroscopy (NIRS) is a noninvasive mode of estimating regional cerebral and renal/mesenteric oxygen saturation (rSO2) and can detect these signals in no blood flow situations as in cardiorespiratory arrest. Cerebral NIRS values can reflect cerebral physiological changes (ie. intracranial tissue oxygenation that can be affected by arterial blood flow, tissue perfusion, and venous drainage) during cardiac arrest, during changes in intracranial pressure, during arrest resolution, and after ROSC.
A scoping review was performed as part of the 2020 ILCOR PLS CoSTR process.1 At present, there is no consensus on a cut-off threshold of regional cerebral oxygen saturation [rSO2] that can be used as an indicator of futility, nor is there a single rSO2 value that can be used as a target during CPR or an argument to continue CPR. Adult literature suggests that a trend in rSO2 is the most useful prognostic indicator, although this has not yet been validated in adults or children. Evidence is currently insufficient for ANZCOR to make a recommendation about use of NIRS in children.
Feedback Devices

CPR feedback or prompt devices are intended to improve CPR quality, probability of ROSC, and survival from cardiac arrest.1 Feedback devices involve technology that can measure various aspects of CPR mechanics, including ventilation rate, chest compression mechanics (eg. depth, rate, recoil), and measures of flow time (CPR fraction, pre- and post-shock pauses). Data can be presented to the provider in real time and/or provided in a summary report at the end of a resuscitation.1
A systematic review on this topic (including adults and children with cardiac arrest) was performed as part of the 2020 ILCOR BLS Taskforce CoSTR process.30
ANZCOR suggests the use of real-time audiovisual feedback and prompt devices during CPR in clinical practice as part of a comprehensive quality improvement program for cardiac arrest designed to ensure high-quality CPR delivery and resuscitation care [weak recommendation, very-low-certainty evidence].1
ANZCOR suggests against the use of real-time audiovisual feedback and prompt devices in isolation (ie. not part of a comprehensive quality improvement program) [weak recommendation, very-low-certainty evidence].1
Checking of resuscitation equipment

ANZCOR guidelines should be considered in conjunction with accepted National Standards and local policies. ANZCOR is aware of cases where equipment failure (eg. resuscitation bag valve devices incorrectly assembled or oxygen supply being incorrectly connected, resulting in hypoxic gases being administered) has led to adverse outcomes.
The checking and maintenance of hospital and resuscitation equipment is covered by National Standards and local policies. Practitioners involved in resuscitation should always be alert to errors of assembly or use and have checking processes to minimise these risks before equipment is used. They should also respond to unexpected situations with further checking procedures. In the case of unexplained hypoxia, this may include changing gas supply and circuits, removing the patient from ventilators and gas supplies, and using a self-inflating bag with room air. In this situation, oxygen analysis of delivered gases should be considered and an oxygen analyser should be available.
Family presence during resuscitation

Current literature supports that family presence during a paediatric resuscitation does not adversely affect survival, nor does it impede the performance of the resuscitation team.
Most studies have reported that family presence during the trauma or cardiac resuscitation of a paediatric family member did not have detrimental emotional or psychological impacts. and that being present at the resuscitation was associated with improved measures of coping and positive emotional outcomes (Refer to ANZCOR Guideline 10.6).
Family members should be kept closely informed of events. They should be provided the opportunity (but not coerced) to be present at the resuscitation of their child. A staff member should be assigned to be with them and support them during the process. If family presence is negatively affecting the performance of the resuscitation by healthcare personnel, the family may sensitively be asked to leave. If resuscitation is unsuccessful or treatment is withdrawn or withheld, parents should be given the opportunity to be with their deceased child after equipment has been removed. If a coronial enquiry is necessary, removal of devices may require permission from a coroner. Follow-up discussion should be routinely offered to parents.
ANZCOR suggests that family members of patients undergoing resuscitation should be given the option to be present, ideally with an assigned support person [Good Practice Statement]. Each healthcare institution should have a family presence policy and staff education strategy in place.
References

- Maconochie IK, Aickin R, Hazinski MF, Atkins DL, Bingham R, Couto TB, Guerguerian AM, Nadkarni VM, Ng KC, Nuthall GA, Ong GYK, Reis AG, Schexnayder SM, Scholefield BR, Tijssen JA, Nolan JP, Morley PT, Van de Voorde P, Zaritsky AL, de Caen AR; Pediatric Life Support Collaborators. Pediatric Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Resuscitation. 2020 Nov;156:A120-A155.
- Van de Voorde P, Turner NM, Djakow J, de Lucas N, Martinez-Mejias A, Biarent D, Bingham R, Brissaud O, Hoffmann F, Johannesdottir GB, Lauritsen T, Maconochie I. European Resuscitation Council Guidelines 2021: Paediatric Life Support. Resuscitation. 2021 Apr;161:327-387.
- Topjian AA, Raymond TT, Atkins D, Chan M, Duff JP, Joyner BL Jr, Lasa JJ, Lavonas EJ, Levy A, Mahgoub M, Meckler GD, Roberts KE, Sutton RM, Schexnayder SM; Pediatric Basic and Advanced Life Support Collaborators. Part 4: Pediatric Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020 Oct 20;142(16_suppl_2):S469-S523.
- Maconochie IK, de Caen AR, Aickin R, Atkins DL, Biarent D, Guerguerian AM, Kleinman ME, Kloeck DA, Meaney PA, Nadkarni VM, Ng KC, Nuthall G, Reis AG, Shimizu N, Tibballs J, Pintos RV. Part 6: Pediatric basic life support and pediatric advanced life support 2015 International Consensus on cardiopulmonary Resuscitation and emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2015: 95: e147-e168.
- de Caen AR, Kleinman ME, Chameides L, Atkins DL, Berg RA, Berg MD, Bhanji F, Biarent D, Bingham R, Coovadia AH, Hazinski MF, Hickey RW, Nadkarni VM, Reis AG, Rodriguez-Nunez A, Tibballs J, Zaritsky AL, Zideman D, On behalf of the Paediatric Basic and Advanced Life Support Chapter Collaborators. Part 10: Paediatric basic and advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2010;81:e213–e259.
- Consensus on Resuscitation Science & Treatment Recommendations. Part 6, Paediatric basic and advanced life support. Resuscitation 2005; 67: 271-291.
- Safranek DJ, Eisenberg MS, Larsen MP. The epidemiology of cardiac arrest in young adults. Ann Emerg Med 1992;21:1102–6.
- Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS; American Heart Association Get With the Guidelines–Resuscitation Investigators. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get With the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes. 2013 Jan 1;6(1):42-9.
- Azzopardi P, Kinney S, Moulden A, Tibballs J. Attitudes and barriers to a Medical Emergency Team system at a tertiary paediatric hospital. Resuscitation. 2011 Feb;82(2):167-74.
- Tibballs J, Russell P. Reliability of pulse palpation by healthcare personnel to diagnose paediatric cardiac arrest. Resuscitation 2009; 80: 61-64.
- Tibballs J, Weeranatna C. The influence of time on the accuracy of healthcare personnel to diagnose paediatric cardiac arrest by pulse palpation. Resuscitation 2010; 81: 671-675.
- Carter BG, Fairbank B, Tibballs J, Hochmann M, Osborne A. Oxygen delivery using self-inflating resuscitation bags. Pediatric Crit Care Med 2005; 6: 125-128.
- Ashoor HM, Lillie E, Zarin W, Pham B, Khan PA, Nincic V, et al. Effectiveness of different compression-to-ventilation methods for cardiopulmonary resuscitation: A systematic review. Resuscitation. 2017; 118:112-25.
- Maconochie I, Aickin R, Atkins D, Bingham B, Chong KC, Couto T, De Van Voorde P, Guerguerian A, Hazinski M, Meaney P, Nadkarni V, Nuthall G, Ong G, Reis A, Shimizu N, Schexnayder S, Tijssen J, De Caen A. CPR : Chest Compression to Ventilation Ratio-Bystander-Pediatric Consensus on Science and Treatment Recommendation [Internet]. Brussels, Belgium: International Liaison Committee on Resuscitation (ILCOR), Pediatric Life Support Task Force, 2017 June 30. Available from: http://www.ilcor.org
- Soar J, Maconochie I, Wyckoff MH, et al. 2019 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2019;145:95-150.
- Sutton RM, Reeder RW, Landis WP, et al. Ventilation rates and pediatric in-hospital cardiac arrest survival outcomes. Crit Care Med 2019;47:1627-36.
- Children’s Hospital of Philadelphia. Quality of Pediatric Resuscitation in a Multicenter Collaborative (pediRES-Q). https://www.clinicaltrials.gov/ct2/show/NCT02708134. Updated April 10, 2019. Accessed August 10, 2020.
- Andersen LW, Berg KM, Saindon BZ, et al. Time to epinephrine and survival after pediatric in-hospital cardiac arrest. JAMA 2015;314:802-10.
- Lin YR, Wu MH, Chen TY, et al. Time to epinephrine treatment is associated with the risk of mortality in children who achieve sustained ROSC after traumatic out-of-hospital cardiac arrest. CritCare 2019;23:101.
- Lin YR, Li CJ, Huang CC, et al. Early epinephrine improves the stabilization of initial post-resuscitation hemodynamics in children with non-shockable out-of-hospital cardiac arrest. Front Pediatr 2019;7:220.
- Hansen M, Schmicker RH, Newgard CD, et al. Time to epinephrine administration and survival from nonshockable out-of-hospital cardiac arrest among children and adults. Circulation 2018;137:2032-40.
- Fukuda T, Kondo Y, Hayashida K, Sekiguchi H, Kukita I. Time to epinephrine and survival after paediatric out-of-hospital cardiac arrest. Eur Heart J Cardiovasc Pharmacother 2018;4:144-51.
- Meert K, Telford R, Holubkov R, et al. Paediatric in-hospital cardiac arrest: Factors associated with survival and neurobehavioural outcome one year later. Resuscitation 2018;124:96-105.
- Hoyme DB, Patel SS, Samson RA, et al. Epinephrine dosing interval and survival outcomes during pediatric in-hospital cardiac arrest. Resuscitation 2017;117:18-23.
- Soar J, Donnino MW, Maconochie I, Aickin R, Atkins DL, Andersen LW, et al. 2018 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations Summary. Resuscitation. 2018;133:194–206.
- Mercier E, Laroche E, Beck B et al. Defibrillation energy dose during pediatric cardiac arrest: systematic review of human and animal model studies. Resuscitation 2019;139:241-52.
- The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 10: pediatric advanced life support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 2000;102:I291-342.
- Wolfe HA, Sutton RM, Reeder RW et al. Functional outcomes among survivors of pediatric in-hospital cardiac arrest are associated with baseline neurologic and functional status, but not with diastolic blood pressure during CPR. Resuscitation 2019;143:57-65.
- Berg RA, SuttonRM, Reeder RW et al. Association between diastolic blood pressure during pediatric in-hospital cardiopulmonary resuscitation and survival. Circulation 2018;137:1784-95.
- Olasveengen TM, Mancini ME, Perkins GD, Avis S, Brooks S, Castrén M, Chung SP, Considine J, Couper K, Escalante R, Hatanaka T, Hung KKC, Kudenchuk P, Lim SH, Nishiyama C, Ristagno G, Semeraro F, Smith CM, Smyth MA, Vaillancourt C, Nolan JP, Hazinski MF, Morley PT; Adult Basic Life Support Collaborators. Adult Basic Life Support: International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Resuscitation. 2020 Nov;156:A35-A79.
About this Guideline

|
Search date/s |
ILCOR literature search details and dates are available on the CoSTR page of the ILCOR website (https://costr.ilcor.org) and the relevant CoSTR documents. |
|
Questions/PICOs: |
Are described in the CoSTR documents (https://costr.ilcor.org) |
|
Method: |
Mixed methods including ARC NHMRC methodology before 2017 and ILCOR GRADE methodology described in ILCOR publications since 2017. The guideline process includes involvement of stakeholders from member organisations of the ARC & NZRC, and peer review by members of the Australian and New Zealand Committee on Resuscitation (ANZCOR). Details of the guideline development process can be found on the ARC website at https://resus.org.au. |
|
Principal reviewers: |
Jason Acworth, Richard Aickin, Gabrielle Nuthall |
|
Main changes: |
This guideline merges content from previous Guidelines 12.1, 12.2, 12.3, 12.4 and elements of 12.6 into a single guideline focused upon the acute resuscitative phase of paediatric ALS. |
|
Approved: |
13 November 2021 |


